RESEARCH
Ruda Lee, Ph.D.
 |
Prof. Ruda Lee, PH.D. Associate Professor International Research Organization for Advanced Science and Technology (IROAST), Kumamoto University, Japan |
Professional Education
Ph.D., Korea University, School of Medicine (2013)
Research training
| 2017-present | Associate Professor (Kumamoto University, Japan) |
|---|---|
| 2014-2016 | Postdoctral fellow (IIT, Italy) |
| 2013-2014 | Postdoctral fellow (KIST, Korea) |
| 2009-2013 | MS & Ph.D course trainee (KIST, Korea) |
| 2008-2009 | Research Assistant (KIST, Korea) |
Selected peer-reviewed publications
- Lee A et al. Early diagnosis of arthritis in mice with collagen-induced arthritis, using a fluorogenic MMP 3-specific polymeric probe. Arthritis Rheum 2011
- Lee A et al. Measurement of MMP Activity in Synovial Fluid in Cases of Osteoarthritis and Acute Inflammatory Conditions of the Knee Joints Using a Fluorogenic Peptide Probe-Immobilized Diagnostic Kit. Theranostics 2012
- Lee A et al. A novel near-infrared fluorescence chemosensor for copper ion detection using click ligation and energy transfer. Chem Commun 2013
- Lee A et al. TNF-α Gene Silencing Using Polymerized siRNA/Thiolated Glycol Chitosan Nanoparticles for Rheumatoid Arthritis. Mol Ther 2014
- Lee A et al. Spherical Polymeric Nanoconstructs for Combined Chemotherapeutic and anti-Inflammatory Therapies. Nanomedicine : NBM 2016
- Lee A et al. Dexamethasone-loaded nanoconstructs for treating and monitoring inflammation in murine colitis models”, Theranostics 2017
Research interest
Research in the Lee lab develops novel molecular imaging sensors and multifunction drug delivery carriers for ‘Theragnosis’ (Therapy + Diagnosis).
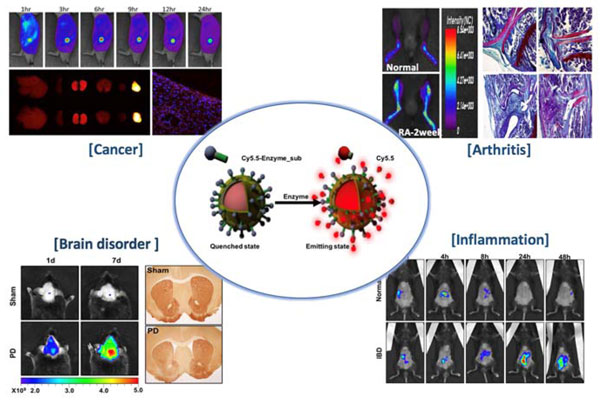
1. Enzyme specific activated imaging sensor based real-time in vivo imaging
Proteases are among the most studied enzyme families due to their involvement in the regulation of diverse disease processes and their potential value as biomarkers and therapeutic targets. We reported various protease target Near-infrared fluorescence (NIRF) imaging sensors and detecting pathologic processes at the cellular and molecular levels in vivo, such as cancer, arthritis, brain disorder and inflammation.
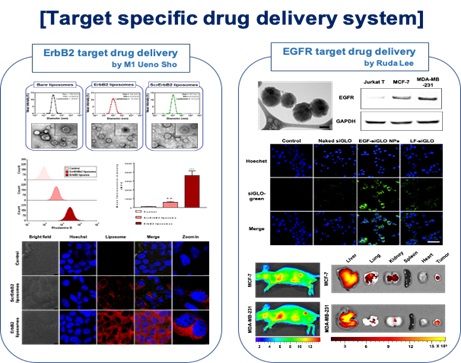
2. Active targeting drug delivery nanomaterials
Active targeted drug delivery material delivers a therapeutic and/or diagnostic agent to a targeted diseased area. Drug-loaded nanomaterials (NPs) are decorated by the peptide, aptamer, antibody for recognition by specific receptors/antigens on target cells. In this strategy, NPs can avoid non-specific biodistribution, reducing cytotoxicity and treatment dosage. It could provide patients to have chances of effective therapeutic treatment.
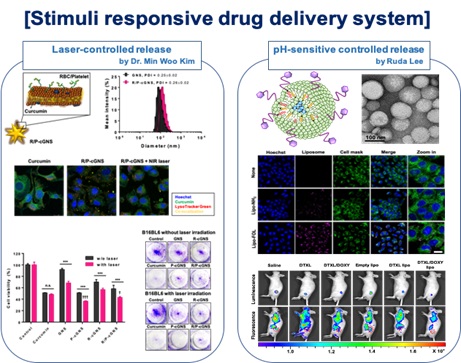
3. Stimuli responsive controlled drug release system
Owing to precise stimuli response, stimuli-responsive DDSs can control drug release, so as to improve the curative effects, reduce the damage of normal tissues and organs, and decrease the side effects of traditional anticancer drugs. We designed endogenous stimuli-responsive materials (pH, enzyme and redox responsive) and exogenous stimuli-responsive materials (such as light and ultrasound).
Lab members
| Associate Prof. | Ruda Lee |
| Post. doc | |
| Students | M1 Sho Ueno |
| B4 Naoki Furutachi | |
| B4 Sho Tanigawa |


